Waived vs Non-Waived Laboratory Tests
Understanding the Difference Between Waived and Non-Waived Tests
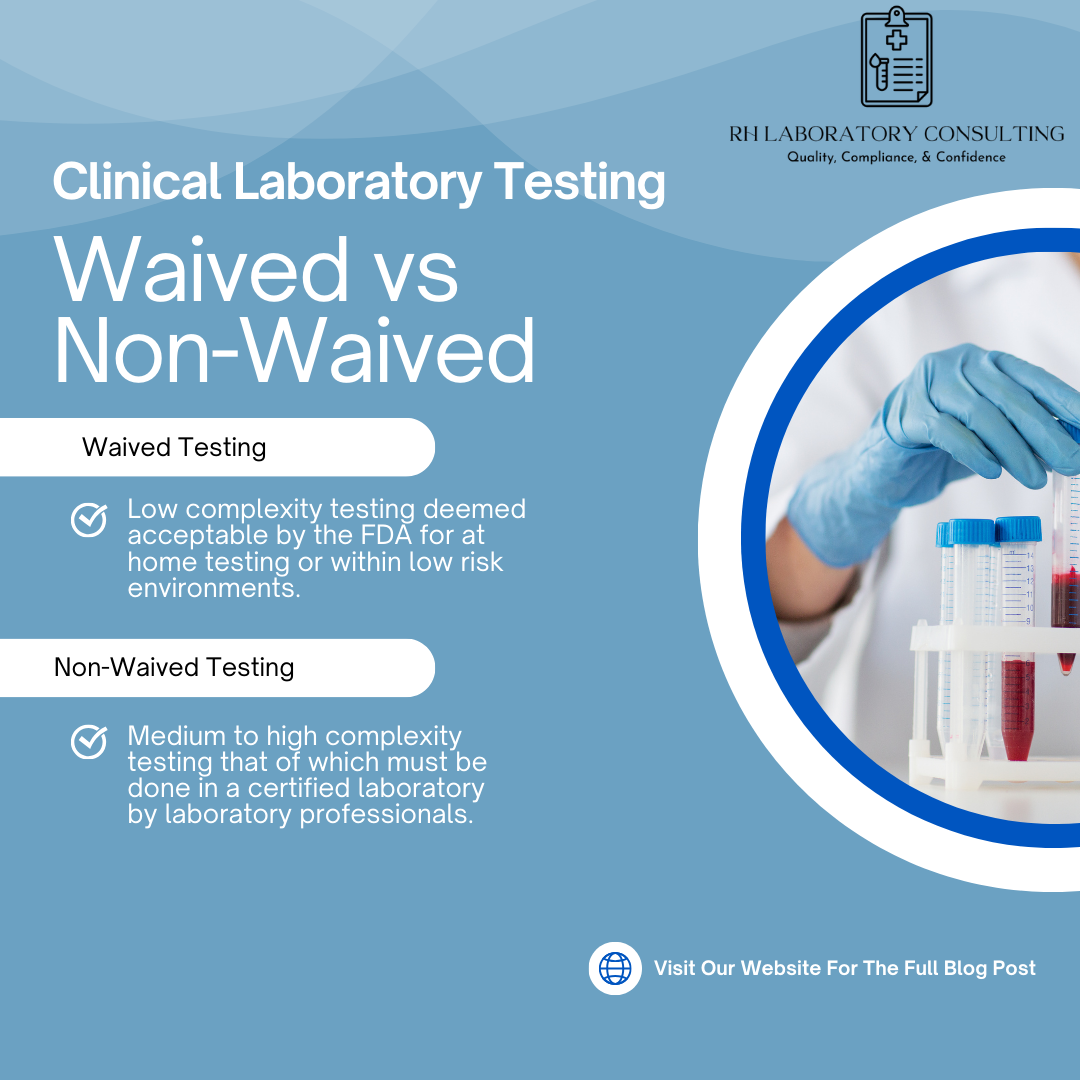
Clinical laboratories perform a wide range of tests, each with specific regulatory requirements. One of the most important distinctions under CLIA is whether a test is classified as waived or non-waived. Understanding this difference is critical for compliance, staffing, quality oversight, and inspection readiness.
Individualized Quality Control Plans
How to Perform an IQCP in Compliance With CAP Requirements
Individualized Quality Control Plans (IQCPs) were introduced to give clinical laboratories more flexibility in how quality control is designed and maintained. Rather than relying solely on rigid, one-size-fits-all QC rules, IQCP allows laboratories to develop a quality plan that reflects how testing truly occurs in their specific environment. When done well, IQCP supports both regulatory compliance and patient safety. When done poorly, it is a common source of CAP inspection findings.